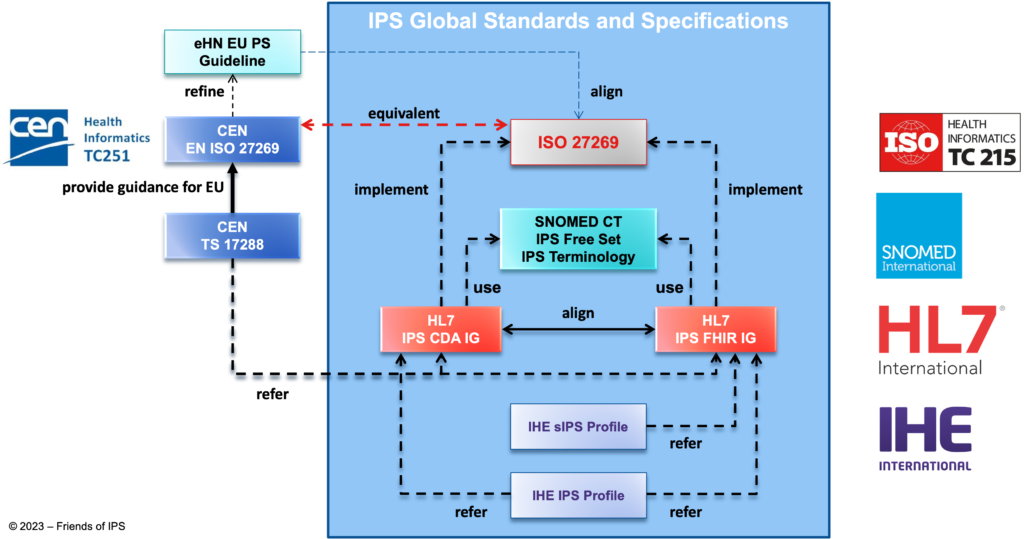
The International Patient Summary (IPS) is building the bridge between the “home” health and care environment of the patient and any other place where the patient needs to visit a clinical professional, whether within or across borders. The construction of the IPS involves a number of standard components and bespoke specifications to make it all work together.
The requirements for the IPS and basic definitions of the IPS data blocks are the subject of the ISO 27269 International Patient Summary standard. This standard is being maintained in close collaboration with the European standards organisation CEN (referred to by CEN as EN ISO 27269) under the Vienna Agreement between ISO and CEN.
HL7 provides two ways to implement the IPS: through the clinical document architecture (CDA), which is the current implementation for cross-border application in Europe, and by using the fast healthcare interoperability resources (FHIR). For both these standards HL7 Implementation Guides (IGs) for the IPS have been developed and are being maintained, the HL7 CDA IPS IG and the HL7 FHIR IPS IG. Basically, these are two equivalent but technically very different implementations of the CEN and ISO IPS standard, using the foundational HL7 CDA and HL7 FHIR standards respectively. They enable the direct exchange and use of structured IPS data between the electronic health record systems in the “home” environment of the patient and elsewhere.
To enable the use of the same coded entries for patient summary sections such as allergies, problems, procedures, and immunizations, SNOMED International makes available the IPS Free Set, under a free creative commons license. In the future, similar subsets may be published by LOINC for diagnostic tests, by DICOM for medical images and by GS1 for medical device or medicinal product identification data to be included in the patient summary. This use of these global code sets and terminologies makes it possible to link directly to existing translations into the local language of the place where the patient is seeking help, so the professionals will be able to understand the clinical history of the patient.
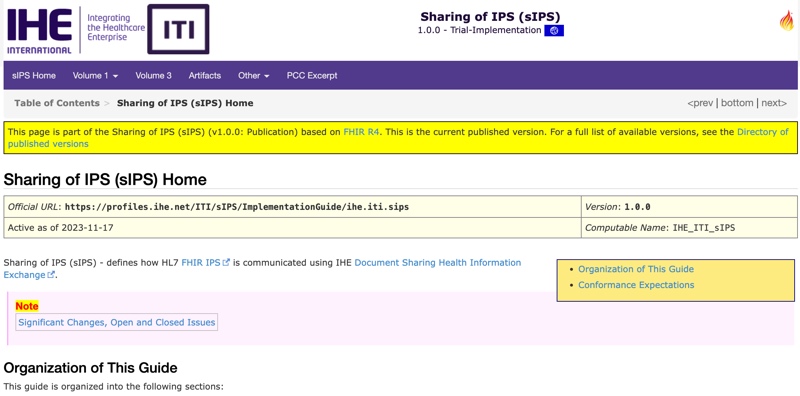
IHE contributes two profiles to support adoption and implementation of the IPS. The IHE sIPS Profile supports the “Sharing of IPS” for those implementers who wish to bind FHIR-based IPS content to existing IHE XDS document-sharing infrastructures, facilitating important functions to support key IPS use cases (publishing an IPS, on-demand access to an IPS, retrieving of an IPS, and pushing an IPS to a recipient). The original IHE IPS Profile, a “content profile”, supports both the FHIR and CDA implementation options, and both profiles add enhanced conformance language to aid implementers in undertaking conformity assessment steps.
Work on the IPS standard originated with the European eHealth Network and their adoption of “Guidelines on Minimum/Nonexhaustive Patient Summary Dataset for Electronic Exchange in accordance with the Cross-Border Directive” (eHN EU PS Guidelines ![]() ) and continuous alignment is an important topic in the maintenance of both the guidelines and the standard.
) and continuous alignment is an important topic in the maintenance of both the guidelines and the standard.
Specifically for the implementation of the IPS in Europe, in line with the cross-border use of the patient summary, CEN has produced technical specification TS 17288. This TS provides guidance on how to implement the specific European requirements which are not applicable throughout the world.
The relationships between the eHN Guideline and the published CEN, ISO, HL7, IHE and SNOMED standards and specifications are summarized and illustrated below.