ISO 27269:2025 – The International Patient Summary Standard
The definition of the International Patient Summary and its constituent IPS Data Blocks is the subject of the ISO 27269 standard. An overview of the IPS Data Blocks is provided in the illustration below.
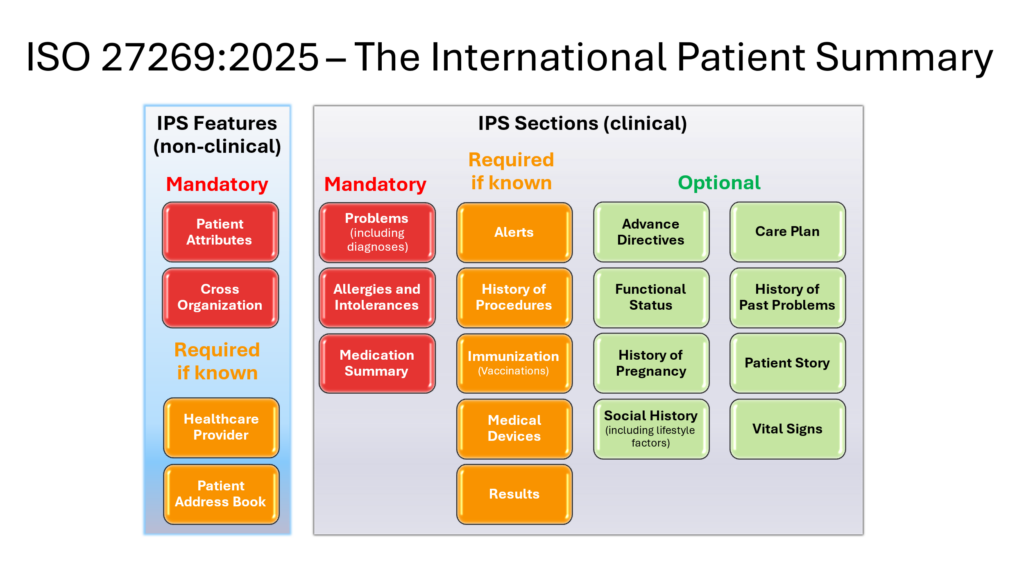
The 2025 version includes new data blocks for “Alerts” and “Patient story”. Also, the “Cross border” and “Provenance” data blocks have now been merged into the new “Cross organization” data block.
Each data block is being described in quite some detail, including purpose, definition, business rules, format, inclusion criteria, currency, and examples. Please note that these technology independent details will not always be represented in the technology dependent implementation guides (such as the HL7 CDA Implementation Guide and the HL7 FHIR Implementation Guide). It is, therefore, advisable to always reference the ISO description in conjunction with the technology specification of your choice.
The ISO IPS standard is available on a global scale as ISO 27269 ![]() and has officially been adopted in Europe as EN ISO 27269
and has officially been adopted in Europe as EN ISO 27269 ![]() . Due to intellectual property and copyright policies of ISO, CEN and their constituent National Standards Bodies, it is not financially possible for these standards to be made available free of charge.
. Due to intellectual property and copyright policies of ISO, CEN and their constituent National Standards Bodies, it is not financially possible for these standards to be made available free of charge.
Some history
CEN TC 251 members agreed that EN 17269 should be fast tracked into ISO, turning the European standard into a fully international one. By doing so, the original CEN IPS project fulfilled its final commitment.
The Fast-track process, began in July 2020, took 3 months, was balloted, and then published by ISO as ‘The International Patient Summary (ISO 27269)’ in 2021. The fast-track process provided clarity and more explanation as well as editorial changes, but it is essentially the same document. The ISO version supersedes EN 17269 as a result, retaining its initial scope focused on the IPS dataset.
The process to make the ISO standard an accepted European norm is under way, and it is expected that it will supersede EN 17269; the ISO standard has officially been adopted as EN ISO 27269 ![]() in Europe in early 2022. Elsewhere, it has already been adopted on a global scale as ISO 27269
in Europe in early 2022. Elsewhere, it has already been adopted on a global scale as ISO 27269 ![]() and all future development on the CEN and ISO IPS standard (i.e., EN ISO 27269) will be carried out jointly within ISO.
and all future development on the CEN and ISO IPS standard (i.e., EN ISO 27269) will be carried out jointly within ISO.
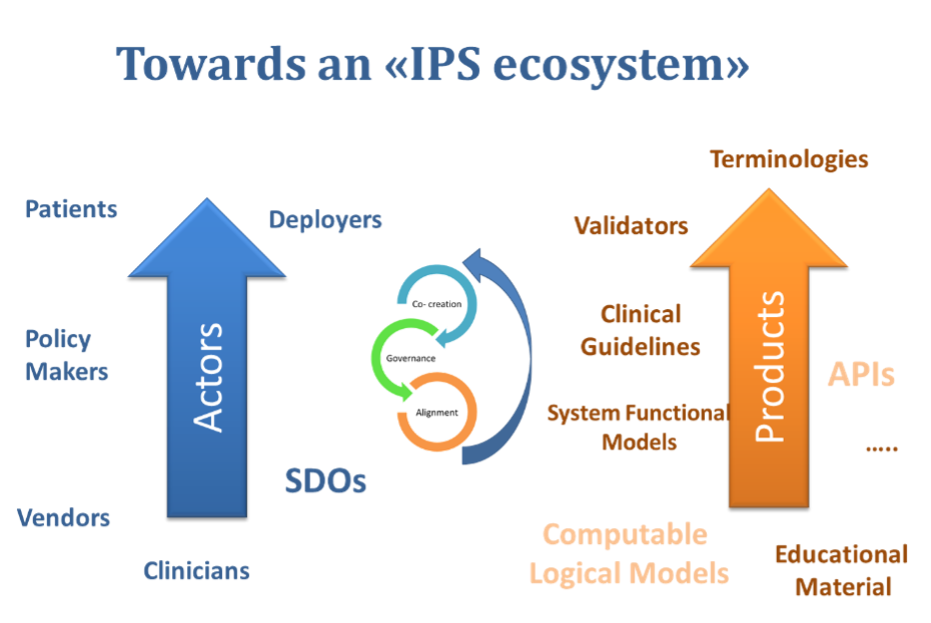
The IPS data model is an important part of the IPS ecosystem, but it is not the whole IPS. The scope is explicit and notes that other services are needed to complement the data model, such as the necessary workflow, the documenting of use cases, or the generic interoperability requirements. The range of models required to offer interoperability, the terminologies, the cardinalities, ontologies, user interfaces and profiles will be required for effective governance to deliver a safe, useful, and usable solution.